- Medical Device – KFDA approval Graft / Prosthesis, Biomaterial
- Non-anaimal Hyaluronic acid – Usage of HA produced by bacterial fermentation(Streptococcus equi) Biocompatibility, Non-Toxin, Non-Immunogenic
- BDDE Crosslinked HA
- BDDE cross-linked HA
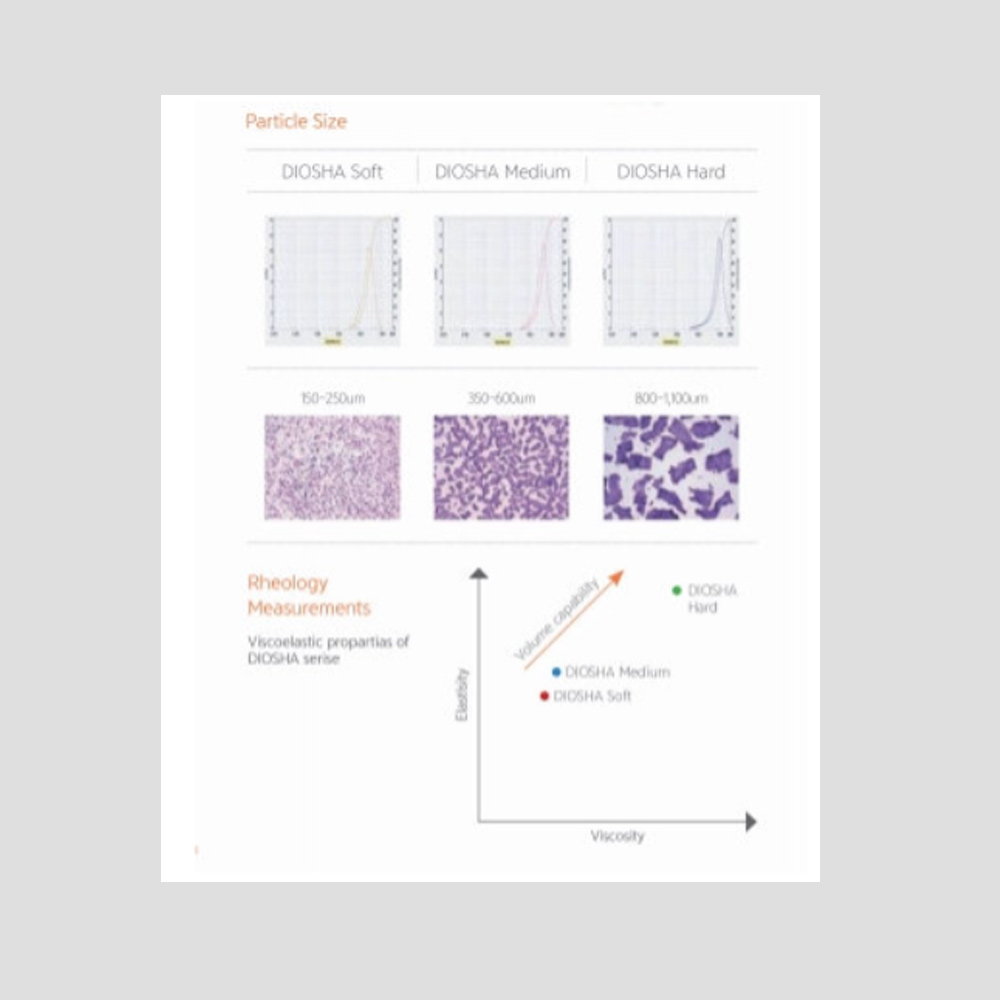
- To overcome the short biodegradability of natural HA
- Improvement of the sustainability
- Proven safety and efficacy – Trough multicenter clinical trial, safety and efficacy of DIOSAH have been proven
Buy 4 get 1, Skin
DIOSHA Dermal Filler (Buy 4 get 1)
$45
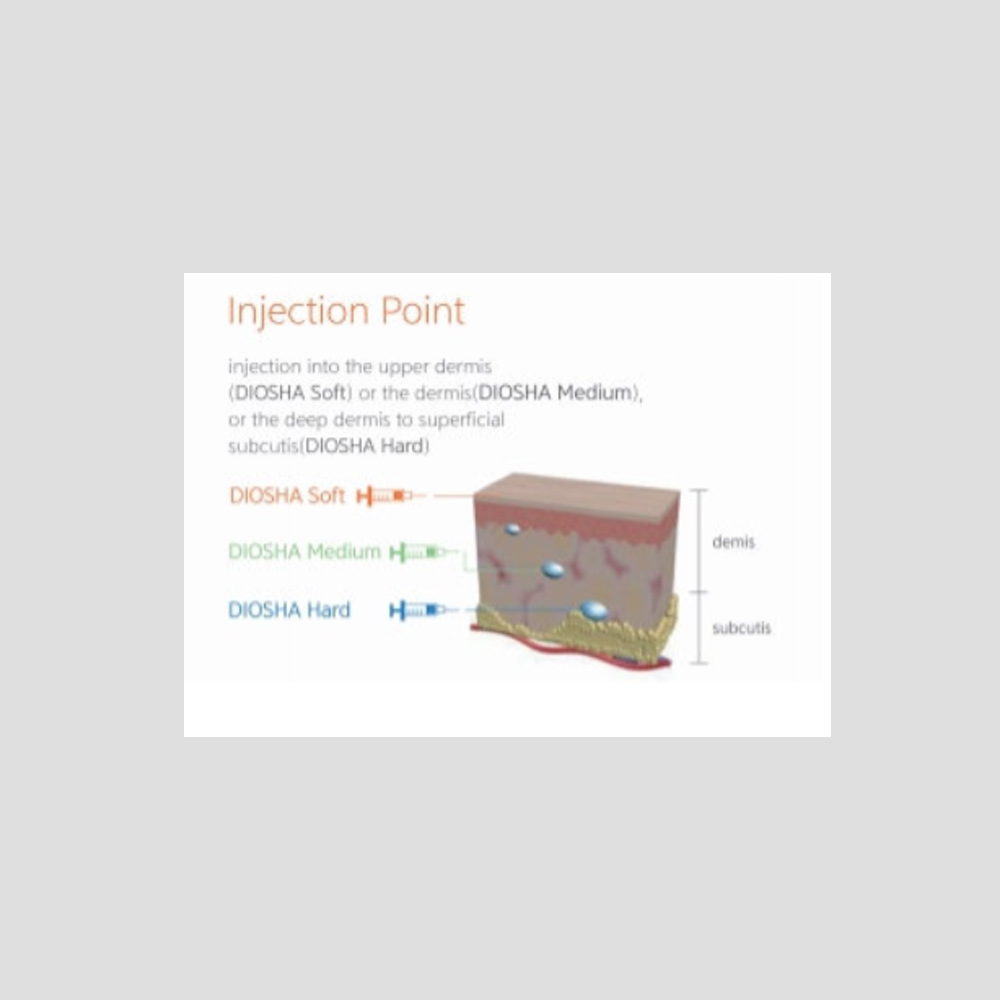
Type : Soft / Medium / Hard
Medical Device – KFDA approval Graft / Prosthesis, Biomaterial
Non-anaimal Hyaluronic acid – Usage of HA produced by bacterial fermentation(Streptococcus equi) Biocompatibility, Non-Toxin, Non-Immunogenic
BDDE Crosslinked HA
BDDE cross-linked HA
To overcome the short biodegradability of natural HA
Improvement of the sustainability
Proven safety and efficacy – Trough multicenter clinical trial, safety and efficacy of DIOSAH have been proven
**CE certified
“Non Lidocaine”
| Type | Hard, Medium, Soft |
|---|












Reviews
There are no reviews yet.